Neonatal Jaundice and Autism: Precautionary Principle Invocation Overdue - Cureus
Recently, a third meta-analysis found neonatal jaundice is associated with substantial possible increased autism risk [1-3] - the latest in a series of repeated warnings of possible long-term neurodevelopmental harm from neonatal jaundice/hyperbilirubinemia, hypernatremia/dehydration, and hypoglycemia, all frequent causes of preventable hospitalizations for insufficient milk intake in breastfed neonates [4,5]. According to a large body of evidence, a sizeable minority of breastfed neonates receive inadequate nutrition/hydration [6-9]. Double-digit percentages of mother-infant dyads do not successfully establish breastfeeding within the first week and milk usually takes days to come in; intensive professional lactation support does not change those facts. The problem is particularly pronounced in first-time mothers, who some estimates suggest experience delayed onset of full milk production (lactogenesis II), with 33-44% of such mothers perceiving milk coming in beyond 72 hours postpartum [9,10]; in another study, Neifert et al. found that 15% of first-time mothers had persistent insufficient milk even after three weeks despite intensive professional lactation support [6].
First-time and more exhausted mothers tend to have more breastfeeding problems, and the risk of autism may be correspondingly greater in first-borns [11-14] and those likely to have more exhausted mothers, including in cases of delivery complications, prolonged labor, breech presentation neonates [13,15], and maternal lupus [16,17]. Additional maternal risk factors for breastfeeding insufficiencies include metabolic conditions such as overweight/obesity [18], gestational diabetes [19], polycystic ovarian syndrome (PCOS), breast tissue abnormalities from hypoplasia (a condition without consensus medical definition that is associated with PCOS and characterized by incomplete breast tissue differentiation) [20], cosmetic surgery [6], birth interventions including Caesarean section, and age. Studies similarly link most of these factors with offspring autism risk [21-25]. More direct links are uncertain due to limited research on breastfeeding problems, particularly with a maternal health emphasis and biological rather than psychosocial orientation. Overall, the available evidence suggests current early infant feeding guidelines incur substantial possible risk for affected neonates [26], as deprivation and frank starvation contribute to preventable jaundice, worsen its severity, and prolong it in the absence of close medical monitoring for near- and full-term neonates (as opposed to their preterm counterparts) - all of which may cause or contribute to brain injury resulting in a continuum of associated neurodevelopmental harms including autism spectrum disorder (ASD) [27,28].
Rising rates of autism [29,30] and other neurodevelopmental disorders [31] coincide with rising breastfeeding rates since the mid-1970s [32-36]. Historically, before its modern resurgence, the pendulum had swung away from breastfeeding in many modern, Western societies for generations. This created intergenerational knowledge gaps about safe breastfeeding. Well-meaning reformers then brought breastfeeding back with a new, historically anomalous emphasis on exclusivity, and in the absence of the pre-existing safety infrastructure - wetnursing [37-41], co-nursing [42], and prelacteal feeding [43-56] - that had protected neonates from common breastfeeding insufficiencies in all prior advanced civilizations. This safety infrastructure persisted in most of the non-Western world, including among the vast majority of foraging societies documented in the Human Relations Area Files (HRAF); in most societies in the HRAF and Standard Cross-Cultural Sample, mothers initiate breastfeeding at least a day and up to a week postpartum [57,58]. This contrasts with the modern, Western ideal of immediate postpartum initiation of birth mother breastfeeding. Where breastfeeding practices incorporating prelacteal feeding and shared nursing norms had been largely replaced by modern formula-feeding, the subsequent introduction of "exclusive breastfeeding" without an attendant safety infrastructure addressing common breastfeeding insufficiencies [26] led to an epidemic of common and preventable harm to neonates from insufficient nutrition/hydration [27,59-67].
The evidence on neonatal jaundice and autism suggests that rising autism rates may be part of that epidemic. As Amin et al. observe, if unconjugated hyperbilirubinemia is a significant cause of autism, it "is easily testable, implies prevention, and is of public health importance." The precautionary principle, acting to prevent harm when risk is uncertain and stakes are high, applies. Neonates should drink appropriate supplemental milk early, adequately, and often. This practice should be standard of care whenever insufficient breastmilk intake is suspected, particularly in cases of jaundiced neonates. This usually entails formula-feeding due to factors including limited banked donor breastmilk availability and infectious disease transmission concerns [26]. Minimizing neonatal deprivation and starvation periods should be standard in research and practice. A link between phototherapy treatment, the current standard treatment for neonatal jaundice, and possible autism risk further underscores the relevance of the precautionary principle, and the urgency of preventing and promptly treating the root cause of modal jaundice - insufficient milk intake in breastfed neonates - to prevent harm.
A PubMed search (1966 through February 4, 2022) for the terms "jaundice," "autism," and "meta-analysis" returned four published systematic reviews and meta-analyses. Of these, the first three comprehensively analyzed the relationship between jaundice and autism in the selected centrally indexed, peer-reviewed literature; this review details, synthesizes, and builds chiefly on their results, which consistently showed a substantial possible association between neonatal jaundice and autism. The fourth search result, a meta-analysis by Lai et al., focused on something different - the association between bilirubin and kernicterus spectrum disorder (KSD) - and limited its analysis of results relating to autism as a dependent variable to one study, which also showed a substantial possible association [68]. These articles, along with a few recent jaundice-autism studies not included in previous meta-analytical literature, suggest that jaundice may increase autism risk.
This review first summarizes the previous meta-analytical findings, which all show substantial possible risk. Second, this review arbitrates disagreement between these previous meta-analyses on the results of publication bias testing, noting common misuse of funnel plot tests for publication bias and presenting results of novel publication bias testing with a more modern tool, p-curve analysis. This analysis shows that results are unaffected by selective reporting (publication bias) and the underlying literature has evidential value. Hence, the jaundice-autism effect may be quite large and the link seems real; but where does the risk come from?
The link between jaundice severity and risk suggests that time matters; preventing jaundice progression may prevent harm. This is consistent with the evidence on global disparities relating to delayed healthcare access. The evidence on subgroup effects and global disparities further suggests that, in order to better understand where the risk comes from, we need to reconceptualize the dependent variable as a broader continuum of harm; existing estimates are likely underestimates, because other neurodevelopmental harms may occur alongside, or instead of, autism and patient-level mortality (survivorship bias) selects against severity. Other identifiable biases also likely bias existing estimates down: mild case inclusion dilutes the effect, study-level exclusion criteria select against severity, and study populations tend to over-represent wealthy, Western populations, under-representing populations likely to suffer disproportionate associated harm.
This body of evidence raises the question: If jaundice increases autism risk, time matters, and autism prevalence has substantially increased in recent history, then why might have untreated jaundice substantially increased in roughly the same period? The resurgence of breastfeeding from historical lows in modern societies, with novel emphasis on exclusivity and in the absence of previously widespread safety infrastructure guarding against common breastfeeding insufficiencies, offers a plausible explanation: neonates accidentally deprived of sufficient nutrition/hydration may suffer permanent harm [26-28].
Two puzzles in the jaundice-autism literature fit this story. First, meta-analyses report weaker associations in the preterm subgroup, a puzzle because preterms are more vulnerable. Different early infant feeding norms in preterms may make them the control group in the exclusive breastfeeding natural experiment. Second, phototherapy, the current standard treatment for neonatal jaundice, is associated with possible increased autism risk of greater magnitude than that associated with jaundice. The prior norm that phototherapy replaced was treating the root cause of modal neonatal jaundice, insufficient milk intake, through feeding neonates early, adequate, and often supplemental milk. Moreover, phototherapy appears to depend on excretion for its efficacy, and possibly safety, which insufficient milk intake compromises; many researchers have raised questions about possible risks associated with phototherapy, including increased autism risk. The evidence is consistent with the possibility of preventable neurodevelopmental harm from untreated insufficient milk intake in neonates treated with phototherapy for jaundice.
Jaundice-autism meta-analyses: consistent findings of substantial possible risk
Amin et al., Jenabi et al., and Kujabi et al. consistently found that jaundice in near and full-term neonates may risk substantial autism increase [1-3]. Their overlapping confidence intervals based on included studies' pooled ORs estimated possible risk increases up to 67%, 68%, and 76%, respectively.
Amin et al. reported the results of a systematic review and meta-analysis on jaundice and autism including 13 studies retrieved from PubMed and MEDLINE databases published until 2009, mostly using retrospective matched case-control designs [1]. In a random-effects model, they estimated that jaundice, as assessed by total serum bilirubin (TSB), is associated with a substantial possible increased risk of autism. Their confidence intervals for the pooled meta-analysis of 13 examined studies (OR 1.43, 95% CI 1.22-1.67) showed a risk increase of 22-67%.
Jenabi et al. reported results of a systematic review and meta-analysis on jaundice and autism, updating and replicating the analysis by Amin et al. They included 21 studies, five cohort studies, and 16 case-control studies published up to April 2018 indexed in PubMed, Scopus, and Web of Science databases. They characterized their analysis's jaundice-autism correlation as "considerable" and, like Amin et al., observed that some studies reported the relationship was dose-responsive. Their confidence intervals for pooled odds ratio estimates (OR 1.35, 95% CI 1.02-1.68) showed a possible risk increase of 2-68%, with a larger possible effect size for the pooled estimated crude than adjusted OR (1.75 (.96-2.54) versus 1.19 (1.07-1.30)), and pooled risk ratio (RR) estimates (RR 1.39 (1.05-1.74)) showing jaundiced neonates are up to 74% more likely to develop autism [2].
Kujabi et al. reported results of a systematic review and meta-analysis on jaundice and autism replicating the core findings of Amin et al. and Jenabi et al. They examined 32 studies published up to February 2019 indexed in Pubmed, Scopus, Embase, Cochrane, and Google Scholar, limiting their analysis to six studies after deeming nine low risk-of-bias. This analysis reiterated that jaundice is associated with a substantial possible increase in later autism [3]. Of the six studies, analysis of the three identified low risk-of-bias cohort studies (RR 1.09, 95% CI .99-1.20) showed a risk decrease of -1% to an increase of 20%, and analysis of the three identified low risk-of-bias case-control studies (OR 1.29, 95% CI .95-1.76) showed a risk decrease of around -1% to increase of up to 76%. These values, coming from the highly restricted sampling of studies the authors considered best, suggest a sizeable possible autism risk increase. Kujabi et al., however, misinterpreted these results as showing "no association" and lack of "convincing evidence."
This set of meta-analytical results is consistent with possible underestimation (downward bias) in the literature. The most restricted meta-analysis of the three yielded the highest risk increase estimates, which might be because studies of lower quality generate more biased estimates. What do the included studies and underlying data demonstrate about the likely valence of bias in the literature? Previous reviews and meta-analyses have bracketed this question.
Amin et al. did not use a structured risk-of-bias or quality assessment to sort studies, a design choice consistent with relevant methodological recommendations. For instance, Greenland and O'Rourke cautioned that such quality scores have tended to poorly predict study results and are commonly misused in meta-analyses that fail to account for the underlying entities they measure (e.g., failing to account for the direction of the induced bias - virtually nullifying the value of bias/quality assessments), treat quality as low- or one-dimensional when it is many, and fail to weigh quality against formal bias-variance trade-off methods such as hierarchical (random-coefficient) meta-regression [69]. Low-dimensional appraisals, they emphasized, are unlikely to ever be adequate.
Building on Amin et al., Jenabi et al. followed the updated Assessing the Methodological Quality of Systematic Reviews (AMSTAR) guidelines [70] for reviews of non-randomized studies by assessing the impact of study-level risk-of-bias on the review results. They used the Newcastle Ottawa Scale (NOS) [71] to assess the quality of analyzed studies and categorized them as low or high quality. They did not consider the likely direction of bias.
Similarly, Kujabi et al. performed quality assessment including using the NOS. They reported being guided in this quality assessment by the Cochrane Handbook for systematic reviews of interventions [72] and the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) checklist [73]. The relevant Cochrane Handbook chapter is Chapter 25: Assessing risk of bias in a non-randomized study [74]. It includes information on a bias assessment tool, Risk Of Bias In Non-randomised Studies - of Interventions (ROBIN-I). This tool "includes an optional component to judge the direction of the bias for each domain and overall," noting that while "for some domains, the bias is most easily thought of as being towards or away from the null... for other domains (in particular confounding, selection bias and forms of measurement bias such as differential misclassification), the bias needs to be thought of as an increase or decrease in the effect estimate to favor either the experimental intervention or comparator compared with the target trial, rather than towards or away from the null." The STROBE checklist similarly includes the item "Discuss both direction and magnitude of any potential bias" [73]. However, instead of discussing direction and magnitude of potential bias, Kujabi et al. noted their quality "assessment (aims) to show the quality of the studies without suggesting how that might influence the effect estimates." Bracketing the valence and substantive import of bias while relying on risk of bias as a binary quality metric leaves open questions about both bias and quality.
Thus, this present review fills a hole in the meta-analytical jaundice-autism literature by addressing previously bracketed questions about likely bias valence and practical significance. It identifies numerous likely underestimation biases: Mild case inclusion dilutes the effect, study-level exclusion criteria and patient-level mortality (survivorship bias) select against severity, other neurodevelopmental harms may occur alongside or instead of autism, and study populations tend to over-represent wealthy, Western populations, under-representing populations likely to suffer disproportionate associated harm. This matters because it suggests an even more substantial possible link between jaundice and preventable harm to neonates than the one consistently found by jaundice-autism meta-analyses, underscoring the sizeable possible magnitude of the effect and the importance of taking the link seriously. It should also be noted a priori that effect estimate bias could cut multiple ways (generating underestimates, overestimates, or both simultaneously along different or related pathways), and that "absence of evidence is not evidence of absence," in Altman's classic configuration [75]. Together, these facts mean that the scientific evidence on the jaundice-autism link, as usual in human affairs and risk estimates, contains uncertainty; this typical uncertainty about risk in a high-stakes context with sufficient evidence mandates preventive measures.
While these previous meta-analyses agreed neonatal jaundice may be associated with substantial autism risk, they disagreed on the interpretation of the results of publication bias tests. So the effect may be quite large, but is it real - or is there evidence that selective results reporting biased the literature?
Significant jaundice-autism findings demonstrate evidential value: results of publication bias testing with p-curve analysis
Meta-analyses often include publication bias tests, assessing the underlying literature for evidence of selective results reporting of significant versus null results (the "file-drawer problem") [76]. All three previous jaundice-autism meta-analyses used funnel plot tests for publication bias. All showed the same resulting pattern of two subgroup clusters, with a top center cluster reflecting lower estimates and a bottom right quadrant cluster reflecting substantially higher ones.
This is consistent with Sterne et al.'s warning that subgroup effects can hide in such plots [77]. Kujabi et al.'s shading suggest the top center cluster includes at least a few of the cohort studies while the bottom-right appears to be composed of case-control studies. This pattern is consistent with the dilution effect from inclusion of mild cases that Amin et al. observed [1]. It would make sense for cohort studies to be disproportionately affected, since population registry-type studies are likely to contain relatively complete universes of cases.
Despite displaying a markedly similar pattern of results, the first two meta-analyses reported divergent publication bias test results from the third. Amin et al. and Jenabi et al. reported no evidence of publication bias, while Kujabi et al. reported that the funnel plot test demonstrates high risk of publication bias in all studies. Kujabi et al. misinterpreted the test results; all three meta-analyses misused the test and none fully reported related statistical test results, which are essential for interpretation.
A best possible use-case for this test would be with randomized controlled trials performed under similar conditions in similar settings, so that the distribution of variance could be expected to be symmetrical but for selective results reporting; by contrast, the jaundice-autism literature presents a use-case closer to the worst possible end of the spectrum, composed of observational studies defining key constructs differently and heterogeneity accordingly high. Thus, funnel plot tests for publication bias are inappropriate in this context, because the correct statistical conditions for using them are absent. The test's fundamental assumption of symmetrical distribution of variance, as in a hypothetical sample of randomized controlled trials, could reasonably be expected to be violated in this observational context of studies with high heterogeneity; but such asymmetry from heterogeneity, as in the case of the established dilution effect likely contributing to the observed subgroup clustering, would not demonstrate risk of publication bias. In addition, the plots all showed visual asymmetry, which does not necessarily indicate statistical asymmetry; formal statistical tests are needed to distinguish the chance appearance of asymmetry from underlying asymmetry [78]. Amin et al. and Jenabi et al. reported performing such tests; Kujabi et al. did not. Finally, neither visual nor statistical funnel plot asymmetry accurately predicts publication bias [79].
Such misuse of funnel plot tests for publication bias is so widespread as to be normal in the medical literature. In a large survey evaluating all Cochrane Database of Systematic Reviews meta-analyses of binary outcomes with three or more studies, Ioannidis and Trikalinos find that most medical journal meta-analyses that use publication bias tests make this mistake: "Statistical conditions for employing asymmetry tests for publication bias are absent from most meta-analyses; yet, in medical journals, these tests are performed often and interpreted erroneously" [80]. This review replicates and extends that result to the jaundice-autism meta-analytical literature.
This problem illustrates and is part of the larger crisis in science, with perverse incentives undermining the knowledge system that is supposed to underpin medicine and public policy [81-84] and prominent calls to change the incentive structure ongoing for decades [85,86]. The persistence of common mistakes, such as misuse of statistical tests including those for significance and publication bias, illustrates both the ongoing nature of the crisis and how easy amelioration might be; retiring statistical significance thresholding and banning misuse of funnel plot tests for publication bias seem like simple reforms for methods experts to recommend and journals to implement.
Ironically, such publication bias tests are intended to address one particular facet of the crisis in science, the reproducibility crisis [87], in which most important results in scientific fields ranging from biotechnology, hematology, and oncology to psychology cannot be replicated. In this context, Bishop suggests publication bias is only one of the "four horsemen" of irreproducibility, with low statistical power, p-value hacking, and HARKing (hypothesizing after results are known) also threatening the integrity of science [88]. This suggests two insights: first, addressing the crisis in science with ever more quantitative rules and checklists seems to sometimes backfire, undermining the integrity of the literature it was intended to promote; observers have long argued it is the underlying incentive structure that must change, and the failure of technical fixes to promote quality science supports that position. And second, while the funnel plot test for publication bias should not be applied to the jaundice-autism literature because the correct statistical conditions for employing it are absent, the other horsemen of irreproducibility could be considered.
The current review considers low statistical power (underpowering) and p-value hacking. But it should also be noted that there may be other, entirely benign reasons for different studies in the underlying jaundice-autism literature producing different effect sizes. For instance, different ways of defining key constructs could generate substantial heterogeneity, as in the mild case inclusion dilution effect. In addition, possible confounding presents challenges to inferring causality but does not threaten the validity of correlational effect estimates. Insufficient milk intake, the modal root cause of neonatal jaundice, also causes hypoglycemia and hypernatremic dehydration, both of which can cause permanent neurological damage; an apparent jaundice-autism link could result in part from these common comorbidities. But different hospital, country-level, or other norms could generate substantial variation in monitoring for and treatment of these conditions, generating outcome differences. In both examples, selective results reporting is not needed to explain the fact that different studies generate different effect estimates.
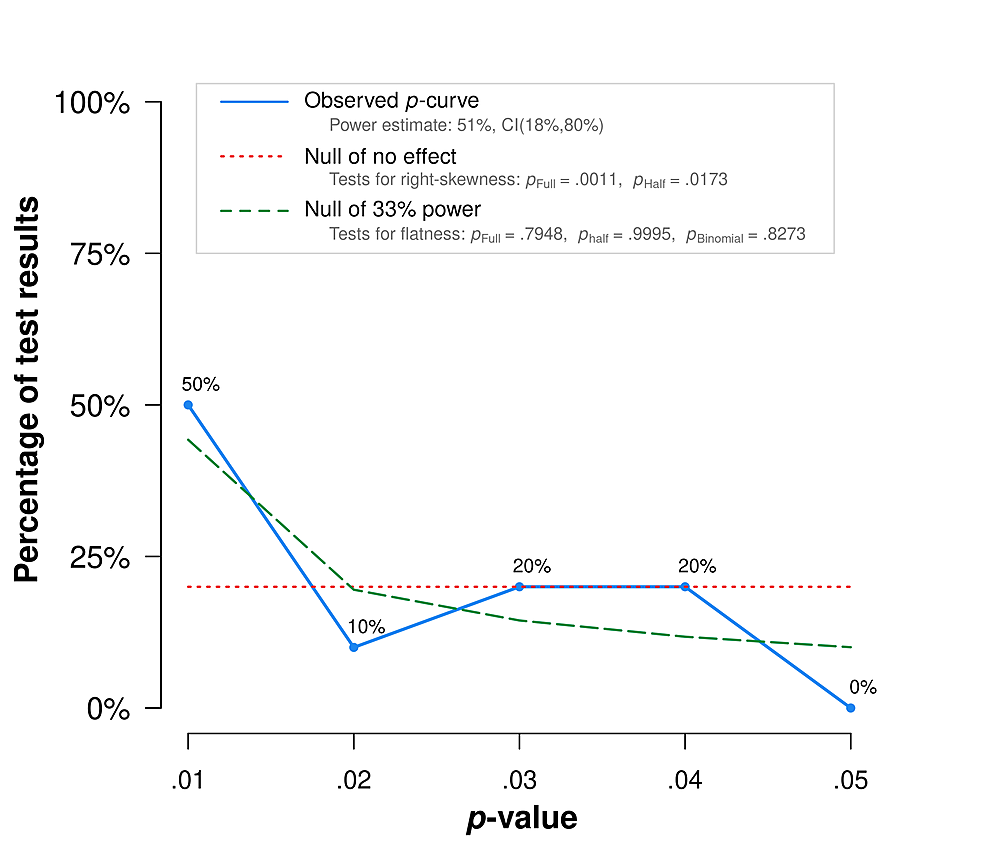
P-curve analysis, a meta-analytical test for evidence of p-value hacking, presents a more modern technique in publication bias testing with relevance to the jaundice-autism literature. It detects selective results reporting by making inferences from the shape of "the distribution of statistically significant p-values for a set of independent findings" [89]. This is important because it lets us know whether a set of significant findings "contains evidential value when we can rule out selective reporting as the sole explanation of those findings." Left-skewed p-curves suggest substantial p-hacking (manipulation of significance testing to find positive results), p-curves that are not skewed suggest lack of evidential value of findings, and right-skewed p-curves "are diagnostic of evidential value" [90]. P-curve testing has numerous limitations, including ignoring nonsignificant results and thus likely producing noisy effect estimates, although this limitation does not make p-curve biased [91], and the method can also bias effect estimates down. These and other limitations [92] suggest p-curve is not an appropriate tool to estimate average effect size. Average effect size estimates should not be interpreted literally in this context anyway, since factors like jaundice severity need to be accounted for, and - a common caveat in scientific evidence reviews relevant to public policy - methodological limitations of available data do not support literal interpretation of point estimates. The appropriate use of this tool in this context is to look at the distribution of p-values, and see if it seems to substantiate or trouble the evidential value of the analyzed literature with respect to potential publication bias, since "p-curve fully corrects for the impact of selectively reporting studies" [91]. So what does the p-curve of jaundice-autism studies show?
This analysis applies Simmons et al.'s study selection guidelines [89], considering for inclusion all PubMed-indexed studies (as of January 3, 2022) that meet published jaundice-autism meta-analysis inclusion criteria (numbering 36). The current review considers more recent studies by Lee et al. [93] and Cordero et al. [94] that previous meta-analyses did not consider, excluding Cordero et al since it does not report p-values. Several studies were excluded because their p-values were statistically non-significant, and p-curve is "the distribution of statistically significant p-values for a set of studies" [89]. Nath et al. is excluded for full-text article inaccessibility [95]. Studies by Sugie et al. [96], Maimburg et al. [97], Finegan-Quarrington [98], Juul-Dam et al. [99], Chien et al., and El baz et al. were excluded for not reporting specific p-values. Lord et al. was excluded due to a lack of jaundice-specific results reporting [100]. This left 10 studies for inclusion in the p-curve analysis (Table 1): Lee et al. [93], Chen et al. [101], Maimburg et al. [102], Bhattarai et al. [103], Lozada et al. [104], Duan et al. [105], Froehlich-Santino et al. [106], Mamidala et al. [107], Zhang et al. [108], and Ahmed et al. [109]. This analysis follows the Official User-Guide to the P-curve [110], including for structuring the P-curve Disclosure Table (Table 1); it generates and reports results using the p-curve app version 4.06 [111].
The p-curve exhibits right-skewness (Figure 1, Table 2). In particular, it meets the combination test that Simonsohn et al. [112] introduced: both the half and full p-curve tests are right-skewed with p<.1. This result suggests the included studies contain evidential value. There is no available evidence that studies linking jaundice and autism demonstrate publication bias. The link warrants further research and action.
So the jaundice-autism effect may be quite large and the statistically significant correlation seems real, but where does the risk come from? The evidence suggests that severity matters; however, there is no safe level of hyperbilirubinemia with respect to neurodevelopmental harm. Both findings indicate that the precautionary principle is particularly relevant to preventing associated harm.
Jaundice severity and autism risk: the case for preventing progression
Time matters; jaundice may already have caused irreversible neurological damage by the time it is identified, particularly when it has progressed. Mandic-Maravic et al. argue damage is done by the time of diagnosis [113]. Bhutani et al. note "once the early stages of hyperbilirubinemic brain damage occur, therapeutic options are limited to the prompt (<8h) use of exchange transfusion, hence the imperative for preventive approaches" in relation to severe neonatal jaundice [114]. Leaving jaundice untreated risks worsening severity, underscoring the need for prompt treatment (or better, prevention) to minimize harm.
Overall, the evidence is consistent with a possible dose-responsive jaundice-autism link wherein greater jaundice severity over a certain threshold poses greater neurodevelopmental risk. In recent reviews and meta-analyses on the jaundice-autism link, Amin et al. and Jenabi et al. noted evidence of a relationship between increased jaundice severity and possible increased autism risk; earlier reviews also presented findings that underscore the importance of preventing jaundice progression in order to prevent possible neurodevelopmental harm. Ip et al. observed that most cases of kernicterus (severe bilirubin-induced brain injury) were associated with bilirubin over 20 mg/dL [115]. Trikalinos et al. found TSB measurement shows reasonable predictive power, but screening does not necessarily improve clinical outcomes [116]; this is consistent with the possibility that damage is done by the time of diagnosis. In a recent systematic review and meta-analysis on the association between bilirubin and KSD, Lai et al. found TSB >25 mg/dL appears to increase the risk of KSD [68]. They conclude in agreement with Ip et al. [117] that other risk factors should be considered in combination with early TSB, consistent with the consensus view that there is no established safe level of elevated bilirubin in part due to subgroup variation in associated risks.
All three previous jaundice-autism meta-analyses (Amin et al., Jenabi et al., and Kujabi et al.) noted high heterogeneity. Amin et al. presented particularly insightful observations on subgroup effects with respect to severity. First, they noted "all five studies that demonstrated significant positive association... defined jaundice based on the magnitude of TSB concentration and evaluated association between ASD and moderate to severe jaundice (TSB > 10 mg/dl). In comparison, the majority of studies that demonstrated no association (OR<1) between jaundice and ASD included infants with any degree of jaundice" [1]. This suggests that worse hyperbilirubinemia correlates with more possible increased autism risk, over a certain severity threshold. Including mild jaundice cases in analyses appears to dilute the effect. Unless jaundice is treated as a non-binary variable or stratified into multiple binary dependent variables according to severity, this dilution from mild case inclusion is likely to bias jaundice-autism effect estimates down, particularly those based on datasets that may appear to be higher-quality because they include a more complete universe of jaundice data, something that Kujabi et al. does not appear to have considered. Second, Amin et al. noted, "most studies that demonstrated significant positive association between jaundice and ASD... were larger and more recent compared to studies that failed to demonstrate a significant association between jaundice and ASD." This suggests that studies with larger sample sizes and thus less vulnerability to Type-II errors from underpowering tend to report more significant effects. These observations are consistent with the possibility that studies demonstrating no jaundice-autism association (or no significant association, often misinterpreted as no association) may be false negatives.
These observations on heterogeneity are consistent with a dose-responsive jaundice-autism link, which is consistent with a causal role of jaundice in autism development via brain injury resulting in bilirubin-induced neurological dysfunction (BIND). BIND is a continuum that may also include attention deficit hyperactivity disorder (ADHD) [118,119], autism, cerebral palsy [120-122], cognitive and developmental delay and disorder [101], epilepsy [123,124], hearing impairment [125,126], kernicterus [27], language disorder, mood disorders [127], and lower IQ and specific learning disorder [28]. Recent trends toward entertaining causal links build on a tradition including Simon's suggestion that autism might be a variant of kernicterus [128] and Shapiro's observation that, as more became known about the neurobiology of kernicterus, definitions evolved to include athetoid cerebral palsy, impaired upward gaze, deafness, auditory neuropathy or dys-synchrony, and subtle BIND, with some suggesting moderate hyperbilirubinemia [129] may increase risk of later ADHD, autism, and Parkinson's disease.
In later research, Amin et al. reviewed the observational evidence linking the BIND spectrum and some comorbid neurodevelopmental disorders, delineating multiple possible biological mechanisms linking jaundice with harm [28]. As they noted, the relationship between neonatal jaundice and neurodevelopmental risk might be better estimated by a broader conceptualization of potentially associated harms to include a continuum rather than one, relatively narrowly operationalized binary outcome. Estimates focusing exclusively on autism are thus likely underpowered to measure the true outcome of interest, preventable harm associated with neonatal jaundice.
Dose-responsive relationships are often non-linear, with risk rising rapidly over a certain exposure threshold, and rare but serious risks affecting a small minority of individuals. Although evidence suggests jaundice severity tends to correlate with increased risk of neurodevelopmental harm, the low base rate of kernicterus, as well as outlying cases of permanent damage from relatively low levels of hyperbilirubinemia, trouble the notion of a clinically relevant linear relationship between bilirubin level and brain injury. The bottom line clinically is that there is no established safe level of neonatal jaundice.
The evidence supports applying the precautionary principle by feeding hungry neonates formula to prevent and treat jaundice and other common complications of insufficient milk intake, which may risk neurodevelopmental harm [26]. Supplementation with water or sugar water does not reduce hyperbilirubinemia [130,131]. Formula lowers bilirubin by inhibiting intestinal reabsorption [132]. Formula-feeding in places with clean water, high literacy, and stable formula access is well established as safe and is likely often also a safer option than prolonged neonatal starvation in lower-resourced settings where medical care access to treat complications of insufficient milk intake tends to be more limited. By contrast, jaundice treatment options such as phototherapy and exchange transfusions are invasive, costly, potentially stressful for neonates and their families, and may incur substantial risk. In infants who are already medically endangered by moderate to severe jaundice, these interventions may be necessary to limit brain injury and disability; but it is uncertain to what extent damage is already done by the time of diagnosis. In any event, these cases are overwhelmingly preventable through supplementing breastfeeding with formula prophylactically, or in response to signs of persistent infant hunger at the latest. Formula-feeding appears safer for neonates than common complications from insufficient milk intake and current standard treatments for them.
Neonatal jaundice severity is risky, and supplementation prevents modal jaundice from progressing by treating its root cause of insufficient milk intake. So does a little bit of supplementation eliminate the risk? Evidence shows that exclusive breastfeeding is the riskiest infant feeding practice with respect to hospitalization for complications of insufficient milk intake [65-67]. Even though some supplementation is better than none when it comes to avoiding the worst outcomes of neonatal starvation, insufficient supplementation remains risky. Shan et al. report rooming-in is associated with increased exclusive breastfeeding, birth weight loss >10% at day three of age, and neonatal admission for phototherapy for hyperbilirubinemia [67]. Both exclusively breastfed and mixed-fed neonates alike in this sample ran increased hospitalization risk under a policy regime designed to promote exclusive breastfeeding in line with Baby Friendly, the WHO/UNICEF program of exclusive breastfeeding promotion. This suggests even supplemented neonates may frequently suffer medically inadequate nutrition/hydration under Baby Friendly-style policies, which educate mothers on benefits but not risks of exclusive breastfeeding and discourage supplementation unless "medically necessary" [135].
There is insufficient evidence to establish which neonates medically require formula and how much is then medically necessary. This is illustrated by preventable hospitalizations for hyperbilirubinemia in Flaherman et al.'s randomized controlled trials limiting or denying early formula supplementation to neonates with excessive weight loss defined as weight loss ≥5% but <10% of their birthweight at 24-48 hours old [136] or being in the ≥75th percentile for weight loss at age at 24-72 hours [137]; denial appears to cause more harm than supplementation, but sometimes the amount of supplement offered is also medically insufficient and hospitalization results. By prioritizing the restriction of formula over ensuring neonates drink sufficient milk, the exclusive breastfeeding paradigm is associated with increased risks for mixed-fed as well as exclusively breastfed neonates. Supplementation should be sufficient to prevent harm, and the evidence establishes neither that neonates lack a homeostatic capacity to regulate milk intake...

Comments
Post a Comment