Association of placental histology and neonatal hematologic ... - Nature.com
Abstract
Objective
The objective of the paper was to investigate how neonatal hematologic outcomes vary by major placental histopathology categories.
Study design
Placental pathology reports from 5263 subjects were coded into individual placental lesions. Infant hematologic data (complete blood count parameters (n = 1945), transfusions, and phototherapy) were compared by placental pathologic phenotype.
Results
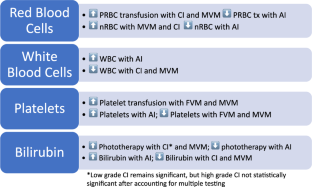
Red blood cell transfusions were more likely with maternal vascular malperfusion (MVM; OR 9.4 [2.2, 40.8]) and chronic inflammation (1.7 [1.04, 2.7]). White blood cells were decreased with MVM (10.6 103/μL vs 16.4) and elevated with acute inflammation (AI; 18.6 vs 11.9). Thrombocytopenia was associated with MVM (OR 3.7 [2.2, 5.1]) and fetal vascular malperfusion (FVM; OR 2.6 [1.5, 4.6]). Platelet transfusions were more likely with MVM (OR 8.3 [4.6, 15.0]) and FVM (OR 2.9 [1.4, 6.1]). Phototherapy was associated with MVM (OR 3.3 [2.7, 4.0]) and AI (OR 0.8 [0.6, 0.9]).
Conclusions
Neonatal hematologic outcomes are associated with the in utero environment described by placental pathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to Journal
Get full journal access for 1 year
118,99 €
only 9,92 € per issue
Tax calculation will be finalised during checkout.
Buy article
Get time limited or full article access on ReadCube.
$32.00
All prices are NET prices.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to potential compromise of individual privacy, but are available from the corresponding author on reasonable request.
References
Lewin S, Bussel JB. Review of fetal and neonatal immune cytopenias. Clin Adv Hematol Oncol. 2015;13:35–43.
Eaves LA, Enggasser AE, Camerota M, Gogcu S, Gower WA, Hartwell H, et al. CpG methylation patterns in placenta and neonatal blood are differentially associated with neonatal inflammation. Pediatr Res. 2022.
Kush ML, Gortner L, Harman CR, Baschat AA. Sustained hematological consequences in the first week of neonatal life secondary to placental dysfunction. Early Hum Dev. 2006;82:67–72.
Ernst LM. Maternal vascular malperfusion of the placental bed. APMIS. 2018;126:551–60.
Voller SB, Chock S, Ernst LM, Su E, Liu X, Farrow KN, et al. Cord blood biomarkers of vascular endothelial growth (VEGF and sFlt-1) and postnatal growth: a preterm birth cohort study. Early Hum Dev. 2014;90:195–200.
Mestan KK, Check J, Minturn L, Yallapragada S, Farrow KN, Liu X, et al. Placental pathologic changes of maternal vascular underperfusion in bronchopulmonary dysplasia and pulmonary hypertension. Placenta. 2014;35:570–4.
Parks WT. Manifestations of hypoxia in the second and third trimester placenta. Birth Defects Res. 2017;109:1345–57.
Parks WT. Placental hypoxia: the lesions of maternal malperfusion. Semin Perinatol. 2015;39:9–19.
Koenig JM, Christensen RD. The mechanism responsible for diminished neutrophil production in neonates delivered of women with pregnancy-induced hypertension. Am J Obstet Gynecol. 1991;165:467–73.
Koenig JM, Christensen RD. Incidence, neutrophil kinetics, and natural history of neonatal neutropenia associated with maternal hypertension. N Engl J Med. 1989;321:557–62.
Wang Y, Gu Y, Philibert L, Lucas MJ. Neutrophil activation induced by placental factors in normal and pre-eclamptic pregnancies in vitro. Placenta. 2001;22:560–5.
Litt JS, Hecht JL. Placental pathology and neonatal thrombocytopenia: lesion type is associated with increased risk. J Perinatol. 2014;34:914–6.
Bester J, Pretorius E. Effects of IL-1beta, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci Rep. 2016;6:32188.
Hom J, Dulmovits BM, Mohandas N, Blanc L. The erythroblastic island as an emerging paradigm in the anemia of inflammation. Immunol Res. 2015;63:75–89.
Melvan JN, Bagby GJ, Welsh DA, Nelson S, Zhang P. Neonatal sepsis and neutrophil insufficiencies. Int Rev Immunol. 2010;29:315–48.
Kuzniewicz MW, Puopolo KM, Fischer A, Walsh EM, Li S, Newman TB, et al. A quantitative, risk-based approach to the management of neonatal early-onset sepsis. JAMA Pediatr. 2017;171:365–71.
American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316.
Khong TY, Mooney EE, Ariel I, Balmus NC, Boyd TK, Brundler MA, et al. Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med. 2016;140:698–713.
Suresh SC, Freedman AA, Hirsch E, Ernst LM. A comprehensive analysis of the association between placental pathology and recurrent preterm birth. Am J Obstet Gynecol. 2022;227:887.e1–887.e15.
Freedman AA, Keenan-Devlin LS, Borders A, Miller GE, Ernst LM. Formulating a meaningful and comprehensive placental phenotypic classification. Pediatr Dev Pathol. 2021;24:337–50.
Haak MC, Oosterhof H, Mouw RJ, Oepkes D, Vandenbussche FP. Pathophysiology and treatment of fetal anemia due to placental chorioangioma. Ultrasound Obstet Gynecol. 1999;14:68–70.
Zook KJ, Mackley AB, Kern J, Paul DA. Hematologic effects of placental pathology on very low birthweight infants born to mothers with preeclampsia. J Perinatol. 2009;29:8–12.
La Gamma EF, Alpan O, Kocherlakota P. Effect of granulocyte colony-stimulating factor on preeclampsia-associated neonatal neutropenia. J Pediatr. 1995;126:457–9.
Joslyn P, Rosenbaum C, Chapple AG, Heard A, Velez M, Barkemeyer B. The effects of maternal hypertension on the early neonatal platelet count. J Perinatol. 2022;42:796–802.
Dubruc E, Lebreton F, Giannoli C, Rabilloud M, Huissoud C, Devouassoux-Shisheboran M, et al. Placental histological lesions in fetal and neonatal alloimmune thrombocytopenia: a retrospective cohort study of 21 cases. Placenta. 2016;48:104–9.
Giesbers S, Bos M, Bulten J, van der Meeren L, van Drongelen J. Subamniotic hemorrhage, a possible new presentation of fetal and neonatal alloimmune thrombocytopenia. Fetal Diagn Ther. 2022;49:60–4.
Macias RI, Marin JJ, Serrano MA. Excretion of biliary compounds during intrauterine life. World J Gastroenterol. 2009;15:817–28.
Jemnitz K, Heredi-Szabo K, Janossy J, Ioja E, Vereczkey L, Krajcsi P. ABCC2/Abcc2: a multispecific transporter with dominant excretory functions. Drug Metab Rev. 2010;42:402–36.
Briz O, Serrano MA, MacIas RI, Gonzalez-Gallego J, Marin JJ. Role of organic anion-transporting polypeptides, OATP-A, OATP-C and OATP-8, in the human placenta-maternal liver tandem excretory pathway for foetal bilirubin. Biochem J. 2003;371:897–905.
Author information
Authors and Affiliations
Contributions
ADF conceptualized and designed the study, assisted with data analysis and interpretation, drafted the initial manuscript, and reviewed and revised the manuscript. AF performed the statistical analysis, assisted with data collection, and reviewed and revised the manuscript. LME conceptualized and designed the study, interpreted data, and reviewed and revised the manuscript. All authors approve the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and Permissions
About this article
Cite this article
Franklin, A.D., Freedman, A. & Ernst, L.M. Association of placental histology and neonatal hematologic outcomes. J Perinatol (2022). https://doi.org/10.1038/s41372-022-01595-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41372-022-01595-z
