Baby Arching Back: Causes, Solutions, and When to Worry
Medications And Their Potential To Cause Increase 'Jaundice Neonatal'
This section presents medications that are known to potentially lead to 'Jaundice neonatal' as a side effect." It's important to note that mild side effects are quite common with medications. Please be aware that the drugs listed here are individual medications and may be part of a broader combination therapy. This information is meant to be a helpful resource but should not replace professional medical advice. If you're concerned about 'Jaundice neonatal', it's best to consult a healthcare professional. In addition to 'Jaundice neonatal', other symptoms or signs might better match your side effect. We have listed these below for your convenience. If you find a symptom that more closely resembles your experience, you can use it to identify potential medications that might be the cause.Advertisement
ropivacaine , medroxyprogesterone , oxytocin Find drugs that can cause other symptoms like 'Jaundice neonatal' Anaemia neonatal , Convulsion neonatal , Death neonatal , Fever neonatal , Grey syndrome neonatal , Hypoglycaemia neonatal , Necrotising colitis , Necrotizing enterocolitis , Necrotising enterocolitis neonatal , Neonatal and infancy disorder , Neonatal disorder , Neonatal hyponatraemia , Neonatal infection , Head lag abnormal , Respiratory disorder neonatal , Sepsis neonatal References
Photo Therapy To Treat Neonatal Jaundice May Cause Moles
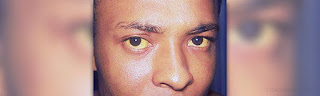
The standard treatment for new born babies who develop jaundice soon after birth, involves exposing them to strong light. French researchers say that this increases the likelihood of moles developing on the skin of the babies later in life. Such moles are termed melanocytic naevi in medical jargon.Since the development of certain skin cancers ,like melanoma ,are linked to naevi, the researchers warn that it is important to ensure that new borns are given sufficient protection when exposing them to intense light therapy.
Advertisement
About 58 children, about 8-9 years of age were studied by researchers .18 of them had received photo therapy after they were born. All the children hailed from the same region in France and were closely akin to each other with respect to colour of hair and eyes and levels of exposure to UV rays. The findings do show an association between development of moles with exposure to light as newborns All children who had been diagnosed with neonatal jaundice when they were babies,and had undergone light treatment for it, developed more moles than those that did not.The naevi that have developed in children who have a history of exposure are about 2-5 mm in diameter, report Dr. Vincent Descamps, from the Bichat-Claude Bernard Hospital in Paris. Similar naevi that developed in kids with no exposure to strong light also happen to be small and of almost the same dimensions. Therefore the results of this study have to be reflected upon with caution. Archives of Dermatology has reported the findings of this current research.
Source-MedindiaMST
Kids Over-the-counter Medicine Recalled For Problem That Can Cause Vomiting, Jaundice
The possibility of "Acetaminophen instability" got all lots of KinderMed Infants' Pain & Fever and KinderMed Kids' Pain & Fever medicine recalled by distributor KinderFarms.
"The two products were manufactured and packaged for KinderFarms by a major, U.S.-based OTC pharmaceutical manufacturer with over 30 years of experience," the recall notice states. "Ongoing testing of sample batches indicated some product lots were no longer in specification and may pose a health risk."
The box for KinderMed Kids' Pain & Fever, 4 fl. Oz./118 ml, with acetaminophen, 160 mg per 5 ml, Oral Suspension FDA
READ MORE: Facility with barefoot workers made Target, CVS, Walmart, Rite Aid eye drops in latest recall
Acetaminophen in the rate of 160 mg per 5 ml is the active ingredient in these KinderMed over-the-counter drugs. If this sounds familiar, in much larger does, it's also the active ingredient in Tylenol and the store brand Tylenol knockoffs.
With the acetaminophen being unstable, "the product may cause acute adverse health effects, including abdominal pain, nausea, vomiting or jaundice at higher doses," the recall notice says, although KinderFarms doesn't know of this happening to anyone yet.
What should parents and caregivers do now?If you have KinderMed Infants' Pain & Fever, UPC No. 850001805698, or KinderMed Kids' Pain & Fever, UPC No. UPC: 850001805728, return them to whomever sold them to you for a full refund.
Questions about this recall should be emailed to consumerrelations@kinderfarms.Com or you can call 800-996-2930, 9 a.M. To 8 p.M., Eastern time.
If there seems to be a medical problem with a child in your care, contact a medical professional. If the problem might have roots in a medication, contact the FDA's MedWatch program either online or by calling 800-463-6332 (press 2 for MedWatch).


Comments
Post a Comment